What is an Ester?
You hear the term “ester” frequently when talking about moonshine and distilling.
Well, what is an ester? How’s it made? And how can it affect my liquor?
Hopefully this short explanation gives you some examples and answers some of those questions.
Let’s start off with something simple first. What is an ester? An ester is just something that has a taste and a smell. They can make things taste sweeter, saltier, or bitter. They can have a variety of smells from musk, fruity, sweet, or even be sour in smell.
For the scientific definition, an ester is an organic compound made by replacing the hydrogen of an acid by an alkyl or other organic group. Many naturally occurring fats and essential oils are esters of fatty acids. And while acetates are a sub class of esters, for the sake of this article, we will only talk about esters themselves.
Ok so now that we have the definitions out of the way, lets talk a little bit about how they’re made.
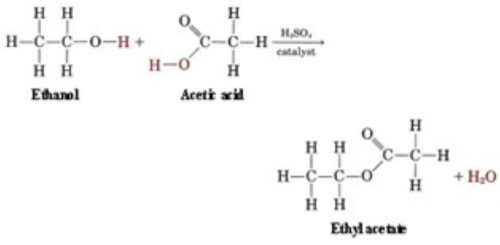
An ester is formed when there is a condensation reaction between an alcohol and carbocyclic acid. Essentially, an acid is bonded with an alcohol. Now that can be any type of acid and any type of alcohol. Some acids are Lactic acid, Acetic Acid, Butyric acid, or Propionic Acid. The alcohol can be anything from ethanol to methanol or propanol. Both alcohols and acids have an oxygen molecule. When bonded together through a condensation reaction, the oxygen molecule on the acid side is removed and replaced with the oxygen molecule from the alcohol. You then end up with an ester.
In a condensation reaction, a pair of molecules join together giving off a small, stable molecule like H2O. In ethyl acetate, as well as others, this small molecule is H2O. Ethanol and acetic acid are joined through condensation reaction. They are separate at first but end up sharing a hydrogen molecule in the end.
Some examples of esters are Ethyl lactate, Ethyl Propanoate, and Ethyl Butyrate.
- Ethyl Lactate is Ethanol joined with Lactic acid, and lactic acid is formed from Lactobacillus which is commonly used in pickles, hot sauces, cheese, yogurt, and many other foods.
- Ethyl Propanoate is another ester which is formed when Ethanol is joined with Propionic Acid. Propionic acid is formed from Propionic Shermanii which commonly used in the making of Swiss Cheese.
- And there’s Ethyl Butyrate, this ester is commonly prized in rum making. It’s formed by joining ethanol with butyric acid which commonly made from Clostridium. And while butyric acid can smell quite foul, the ester it produces makes quite a transformation and can smell like fruits such as pineapples and can be quite pleasant on the nose.
So how can esters affect my final spirit?
Well, if you’re looking for a creamy taste in a whiskey you might want to some ethyl lactate as it can make the whiskey taste and smell creamy. If you’re working with rum, you may want Ethyl Propanoate which in and of itself can smell like a “Fake Rum”. Or you can encourage Ethyl Butyrate which can give you those fruity smells.
Hopefully this demystified what some of those esters are, how they’re made, and how they come through your final product.
But if it raised more questions you would like for me to answer, please feel free to email me at [email protected]
-Moonshine Shua
SOURCES & RESOURCES:
8.18: Esters – Chemistry LibreTexts
How esters are formed YouTube videos
(2) Introduction to Esters – YouTube
(2) Perfumery raw materials – Esters – YouTube
How esters are formed
Preparation of Esters – Chemistry LibreTexts
Please check me out on TikTok @moonshine_shua and UN-TAXED Podcast. If you would like to see me write about something specific, please feel free to contact me at [email protected] or through social media. Thanks!
Thoughts and opinions expressed in this article are that of the author ONLY and are not representative of any business, entity or individual listed therein

Cool. I spent a long time looking for relevant content and found that your article gave me new ideas, which is very helpful for my research. I think my thesis can be completed more smoothly. Thank you.